Manufacturing and Pathogen Safety
Octapharma has pioneered a number of safety innovations that have set the standard for the protein products industry
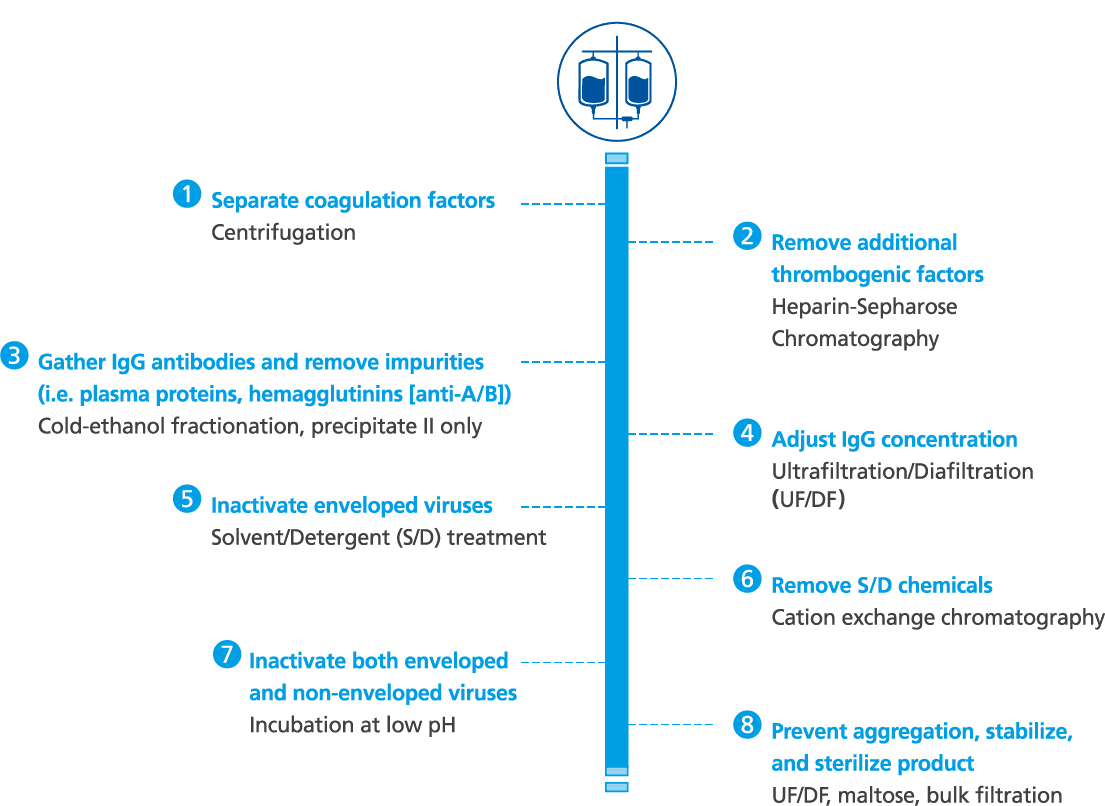
Octagam 5% manufacturing and purification process1-3
octagam 5% contains all 4 subclasses of IgG, with a percentage distribution equivalent to the one found in normal plasma
- Octagam 5% is made from large pools of donated plasma which go through a rigorous purification process for the inactivation and removal of viruses
- Other precautions against viral transmission include: selection of plasma donors, screening of donations and plasma pool, as well as final product testing for viruses
- Octagam 5% is manufactured by cold ethanol fractionation followed by ultrafiltration and chromatography. The manufacturing process includes treatment with a solvent/detergent, and incubation at low pH
Validated viral removal and inactivation process
The manufacturing process for octagam 5% has been validated for its capacity to remove and inactivate both enveloped and non-enveloped viruses1
All units of human plasma used in the manufacture of octagam 5% liquid are collected in US-based Octapharma Plasma centers that are licensed by the US Food and Drug Administration (FDA). Plasma is tested by FDA-licensed serological tests for HBsAg, antibodies to HCV and HIV, and Nucleic Acid Test (NAT) for HCV and HIV-1 and found to be non-reactive (negative).
| Production Step | In vitro reduction factor [log10] | ||||
|---|---|---|---|---|---|
| Enveloped Viruses | Non-Enveloped Viruses | ||||
| HIV-1 | PRV | SBV | MEV | PPV | |
| Cold ethanol fractionation | ≥ 4.81 | ≥ 6.28 | ≥ 7.13 | ≥ 7.13 | ≥6.53 |
| S/D treatment | ≥ 4.93 | 5.23 | ≥ 6.77 | n.a. | n.a. |
| pH 4 treatment | ≥ 4.33 | ≥ 6.71 | 6.71 | 5.07 | < 1* |
| Global reduction factor | ≥ 14.07 | ≥ 18.22 | ≥ 20.61 | ≥ 12.20 | ≥6.53 |
*Not calculated for global LRF.
HIV-1: Human Immunodeficiency Virus-1; PRV: Pseudorabies Virus, model virus for e.g. Hepatitis B Virus (HBV); SBV: Sindbis Virus model virus for Hepatitis C Virus (HCV); MEV: Mouse Encephalomyelitis Virus, model virus for Human Parvovirus B19; PPV: Porcine Parvovirus, model virus for Hepatitis A Virus (HAV)
References:
- Octagam 5% Full Prescribing Information. Paramus, NJ: Octapharma; rev April 2022.
- Octapharma. Data on file.
- Buchacher A, Kaar W. Intravenous immunoglobin G from human plasma—purification concepts and important quality criteria. In: Bertolini J, Goss N, Curling J, eds. Production of Plasma Proteins for Therapeutic Use. Hoboken, NJ: John Wiley & Sons, Inc; 2013.