ProDERM Study Design: IVIg Therapy for Adults With DM
The First and Only IVIg Therapy With a Successful Phase 3 Study Resulting in FDA Approval for Dermatomyositis in Adults1-3
ProDERM is a multicenter, prospective, double blind, randomized clinical trial to evaluate the long-term efficacy, tolerability & safety of IVIg in a large multinational population of adults with DM.
- Enrolled 95 adult patients from 36 sites in 10 countries (17 sites in the US)
- Included patients who were receiving standard immunosuppression (corticosteroids and/or immunosuppressants) or who previously failed or were intolerant to standard immunosuppression
- Study design allowed patients to switch treatment if they deteriorated
- 47 subjects received octagam 10% and 48 subjects received placebo in an initial 16‑week, double-blinded First Period, followed by a 6-month, open-label Extension Period, during which all subjects who were eligible to continue received octagam 10% every 4 weeks. Forty-five subjects in the initial octagam group and 46 subjects in the placebo group entered the Extension Period
- Enabled recruitment of a large number of patients for such a rare disease
- Large patient population helps make results applicable in clinic practice, given the heterogenous nature of DM
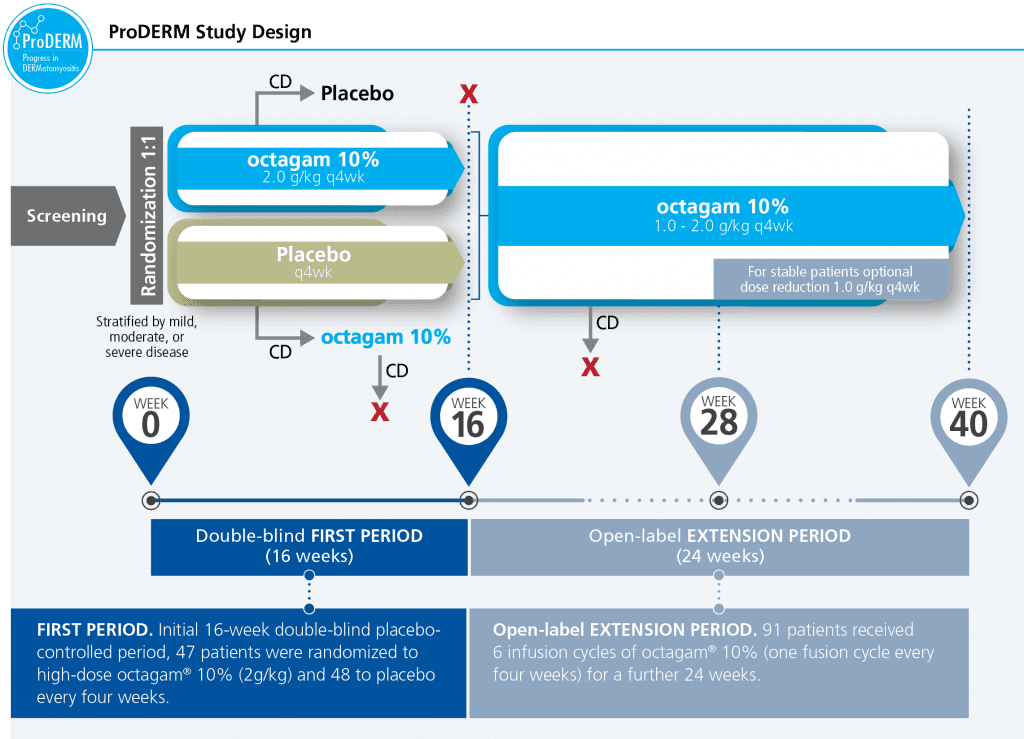
CD=confirmed deterioration.
Patient Demographics1-3
 Inclusion Criteria
Inclusion Criteria
Patients who met the following criteria were eligible for the study:
- Males or females ≥18 to <80 years of age
- Diagnosis of definite or probable DM according to the Bohan and Peter criteria
- Patients under treatment with:
- Corticosteroids and/or maximally 2 immunosuppressants and being on stable therapy for at least 4 weeks
OR - Previous failure of response or previous intolerance to corticosteroid and at least 1 additional immunosuppressive drug, and with steroid/immunosuppressive drugs washed out
- Corticosteroids and/or maximally 2 immunosuppressants and being on stable therapy for at least 4 weeks
- Patients with active disease, assessed and agreed upon by the Independent Adjudication Committee (IAC)
- Manual Muscle Testing-8 (MMT-8) score <142, with at least 2 other abnormal core set measures (Visual Analogue Scale [VAS] of patient global activity ≥2 cm, physician’s global disease activity (GDA) ≥2 cm, extra-muscular activity ≥2 cm; at least one muscle enzyme >1.5 times upper limit of normal, Health Assessment Questionnaire [HAQ] ≥0.25)
 Patients were Typical of a DM Population Encountered in Clinical Practice
Patients were Typical of a DM Population Encountered in Clinical Practice
Demographic characteristics were balanced across octagam 10% and placebo.
- Patient age ranged from 22 to 79 years, with a mean age of around 53 years
- Approximately 90% of patients were Caucasian
- Overall, the majority of patients were female: around 75% vs 25% males
- Approximately 70% of patients were enrolled at sites outside of the US
- The median time since diagnosis of DM was similar in both treatment groups: 2.35 years in the octagam 10% group; 2.86 years in the placebo group
- The majority of patients (70.5% overall) were classed as having definite DM, the other 29.5% as having probable DM
- Mean time since diagnosis of DM was around 4.5 years (octagam 10% 5.3 years, placebo 3.9 years)
- All patients (100%) had muscle weakness and typical DM rash, and more than 90% of patients had elevated muscle enzymes
| Parameter N (%) | octagam 10% (n=47) | Placebo (n=48) | Total (N=95) |
|---|---|---|---|
| Median Age | 55 years | 51.5 years | 52 years |
| Female | 36 (76.6%) | 35 (72.9% | 71 (74.7%) |
| Male | 11 (23.4%) | 13 (27.1%) | 24 (25.3%) |
| Median Time Since DM Diagnosis | 2.35 years | 2.86 years | 2.57 years |
| Bohan and Peters Criteria | |||
| Proximal muscle weakness | 47 (100%) | 48 (100%) | 95 (100%) |
| Muscle biopsy evidence of myositis | 23 (48.9%) | 23 (47.9%) | 46 (48.4%) |
| Elevation of muscle enzymes | 43 (91.5%) | 44 (91.7%) | 87 (91.6%) |
| EMG evidence of myositis | 31 (66%) | 26 (54.2%) | 57 (60%) |
| Typical DM skin rash | 47 (100%) | 48 (100%) | 95 (100%) |
| Classification of DM | |||
| Definite | 34 (72.3%) | 33 (68.8%) | 67 (70.5%) |
| Probable | 13 (27.7%) | 15 (31.3%) | 28 (29.5%) |
| Physician Global Disease Activity | |||
| Mild | 11 (23.4%) | 15 (31.3%) | 26 (27.4%) |
| Moderate | 29 (61.7%) | 27 (56.3%) | 56 (58.9%) |
| Severe | 7 (14.9%) | 6 (12.5%) | 13 (13.7%) |
| Premedication for Infusion | |||
| Any | 10 (21.3%) | 4 (8.3%) | 14 (14.7%) |
| Analgesics | 5 (10.6%) | 1 (2.1%) | 6 (6.3%) |
| Antihistamines | 4 (8.5%) | 2 (4.2%) | 6 (6.3%) |
|
EMG=electromyogram. |
|||
 Concomitant Medication Use
Concomitant Medication Use
- 98.9% of patients continued on their stable prior DM medications
- A similar proportion of patients used concomitant DM medications in both treatment arms. The most common were: systemic corticosteroids (88%); immunosuppressants (57%); and anti-inflammatory and anti-rheumatic products (21%)
Study Endpoints1-3
PRIMARY ENDPOINT: Efficacy was based on proportion of patients responding to treatment at Week 16, defined as improvement of ≥20 points on Total Improvement Score (TIS). TIS is measured on a scale of 0 to 100 (20=minimal; 40=moderate; 60=major), and is based on 6 core set measures:
- Physician global disease activity (GDA). Part of Myositis Disease Activity Assessment Tool (MDAAT) assessed by Investigator on Visual Analog Scale (VAS)
- Manual muscle testing MMT-8. Evaluation of strength in a muscle or muscle group based on performance of a movement
- Muscle enzymes, Aldolase, creatine kinase, alanine aminotransferase, aspartate aminotransferase, lactate dehydrogenase)
- Patient global disease activity (GDA). Assessed by the patient on a VAS
- Health Assessment Questionnaire (HAQ). Assessed by the patient
- Extramuscular disease activity. Part of MDAAT assessed by investigator on VAS
SECONDARY ENDPOINTS:
- Proportion of TIS responders with at least moderate or major improvement
- TIS responders at 40 weeks
- Mean TIS at the end of placebo-controlled period (4 months) and at the end of the open-label extension period (10 months)
- Cutaneous Dermatomyositis Disease Area and Severity Index (CDASI)
- Mean change at 4 months and 10 months
- Assesses erythema, scale, and erosion/ulcerations over 15 areas, including:
scalp, face, neck, upper back and shoulders, buttocks, arms, abdomen, hands, thigh, legs and feet - Total scale of 0-100 (lower scores indicate lower disease severity)
- Safety and tolerability
References:
- Octagam 10% Full Prescribing Information. Paramus, NJ: Octapharma; rev March 2022.
- Octapharma. Data on file.
- Aggarwal R, et al. (ProDERM study). Submitted for publication. August 2021.