- Thrombosis may occur with immune globulin intravenous (IgIV) products, including octagam 10%. Risk factors may include: advanced age, prolonged immobilization, hypercoagulable conditions, history of venous or arterial thrombosis, use of estrogens, indwelling central vascular catheters, hyperviscosity, and cardiovascular risk factors. Thrombosis may occur in the absence of known risk factors.
- Renal dysfunction, acute renal failure, osmotic nephrosis, and death may occur in predisposed patients who receive IGIV products, including octagam 10%. Patients predisposed to renal dysfunction include those with a degree of pre-existing renal insufficiency, diabetes mellitus, age greater than 65, volume depletion, sepsis, paraproteinemia, or patients receiving known nephrotoxic drugs. Renal dysfunction and acute renal failure occur more commonly in patients receiving IGIV products containing sucrose. Octagam 10% does not contain sucrose.
- For patients at risk of thrombosis, renal dysfunction or acute renal failure, administer octagam 10% at the minimum dose and infusion rate practicable. Ensure adequate hydration in patients before administration. Monitor for signs and symptoms of thrombosis and assess blood viscosity in patients at risk for hyperviscosity.
Indications
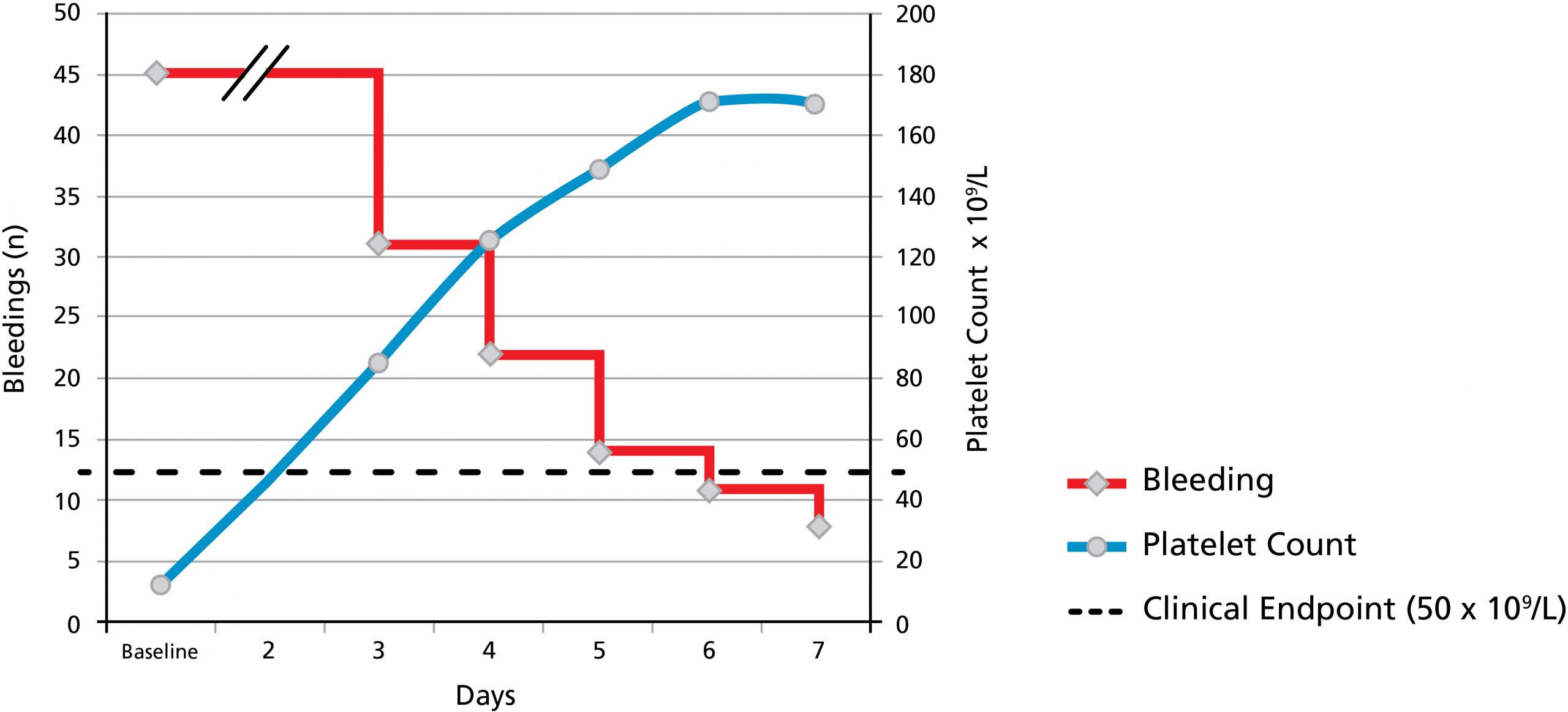
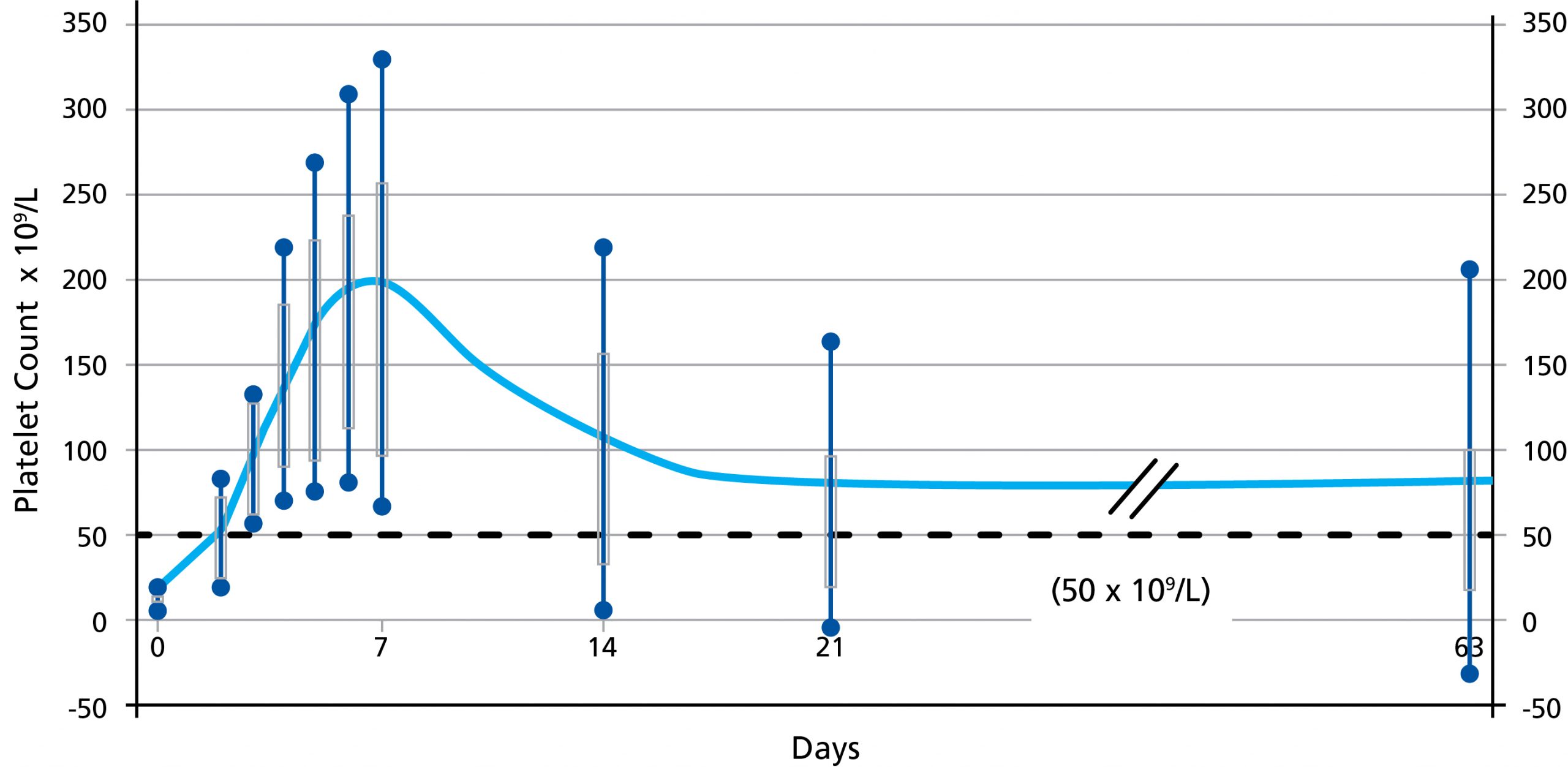
Octagam 10% is an immune globulin intravenous (human) liquid preparation indicated for the treatment of dermatomyositis (DM) in adults. Octagam 10% is also indicated for the treatment of chronic immune thrombocytopenic purpura (ITP) to rapidly raise platelet counts to control or prevent bleeding in adults.
IMPORTANT SAFETY INFORMATION
Dosing and Administration
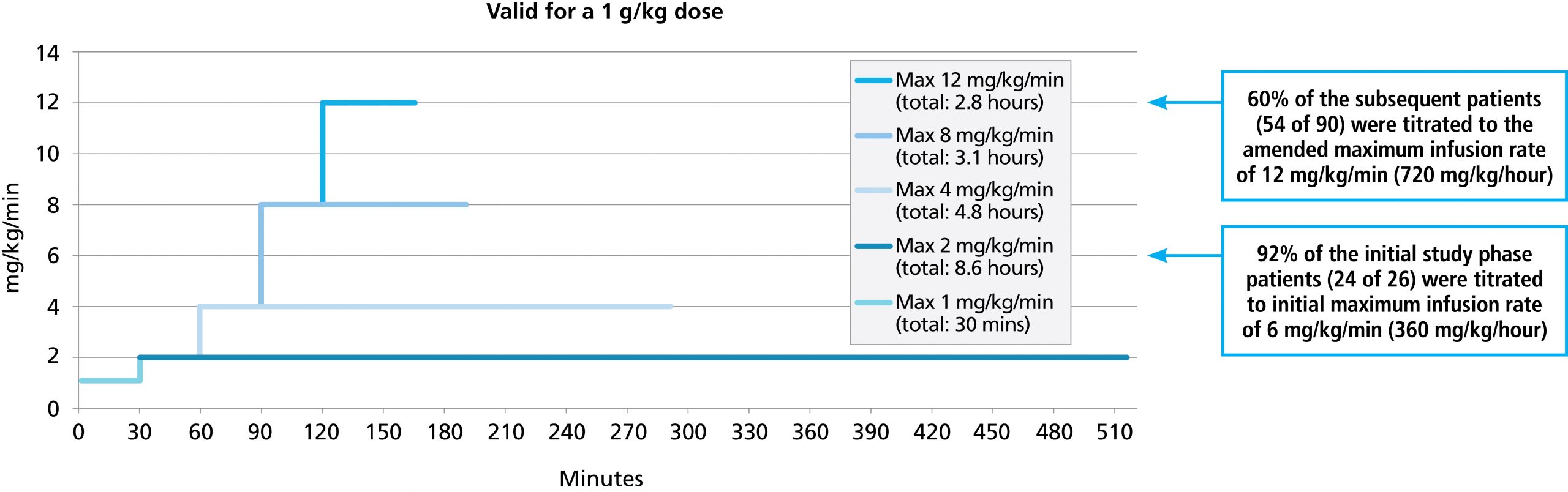
Patients with dermatomyositis are at increased risk for thromboembolic events; monitor carefully and do not exceed an infusion rate of 0.04 mL/kg/min.
Contraindications
Octagam 10% is contraindicated in patients who have a history of severe systemic hypersensitivity reactions, such as anaphylaxis, to human immunoglobulin. Octagam 10% contains trace amounts of IgA (average 106 μg/mL in a 10% solution). It is contraindicated in IgA-deficient patients with antibodies against IgA and history of hypersensitivity.
Warnings and Precautions
IgA-deficient patients with antibodies against IgA are at greater risk of developing severe hypersensitivity and anaphylactic reactions to octagam 10%. Epinephrine should be available immediately to treat any severe acute hypersensitivity reactions.
Monitor renal function, including blood urea nitrogen and serum creatinine, and urine output in patients at risk of developing acute renal failure.
Falsely elevated blood glucose readings may occur during and after the infusion of octagam 10% with testing by some glucometers and test strip systems.
Hyperproteinemia, increased serum osmolarity and hyponatremia may occur in patients receiving octagam 10%.
Hemolysis that is either intravascular or due to enhanced red blood cell sequestration can develop subsequent to octagam 10% treatments. Risk factors for hemolysis include high doses and non-O-blood group. Closely monitor patients for hemolysis and hemolytic anemia.
Aseptic meningitis syndrome may occur in patients receiving octagam 10%, especially with high doses or rapid infusion.
Monitor patients for pulmonary adverse reactions (transfusion-related acute lung injury [TRALI]).
Octagam 10% is made from human plasma and may contain infectious agents, e.g. viruses and, theoretically, the Creutzfeldt-Jakob disease agent.
Adverse Reactions
Chronic Immune Thrombocytopenic Purpura (ITP)
The most common adverse reactions observed in >5% of clinical study subjects with ITP were headache, fever, and increased heart rate.
Dermatomyositis (DM)
The most common adverse reactions observed in > 5% of clinical study subjects with DM were headache, fever, nausea, vomiting, increased blood pressure, chills, musculoskeletal pain, increased heart rate, dyspnea, and infusions site reactions.
Please see octagam 10% full Prescribing Information